A Novel Method for Host Cell Protein Analysis
Gene therapy is a therapeutic approach that involves the delivery of genetic material into a patient's cells to treat or prevent disease. The goal of gene therapy is to correct or compensate for genetic mutations, introduce new genes, or modulate gene expression to restore normal cellular function. This innovative treatment has the potential to address a wide range of diseases, including genetic disorders, cancers, and acquired diseases like HIV/AIDS.
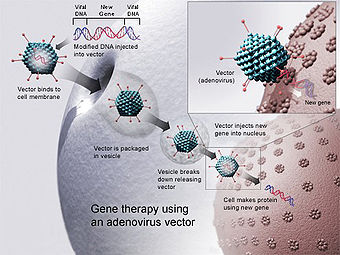
Here's how gene therapy typically works:
Identifying the Target Gene:
The first step in gene therapy is to identify the specific gene or genes associated with the disease being targeted. This may involve genetic testing to determine the underlying genetic cause of the disorder.
Vector Selection:
Gene therapy utilizes vectors, which are vehicles for delivering genetic material into target cells. Vectors can be viruses (such as adenoviruses, lentiviruses, or adeno-associated viruses) or non-viral vectors (such as plasmids or nanoparticles). These vectors are modified to carry the therapeutic gene and to ensure safe and efficient delivery into the target cells.
Delivery:
The vector carrying the therapeutic gene is introduced into the patient's body through various administration routes, depending on the target tissue or cells. This can include direct injection into specific tissues, intravenous infusion, or ex vivo manipulation of cells followed by reinfusion into the patient.
Expression of Therapeutic Gene:
Once the vector enters the target cells, it delivers the therapeutic gene to the cell's nucleus. The therapeutic gene then integrates into the cell's DNA or remains as an episome (in the case of non-integrating vectors). The cell then begins to produce the therapeutic protein encoded by the introduced gene.
Therapeutic Effect:
The expressed therapeutic protein can correct the underlying genetic defect, compensate for the missing or dysfunctional protein, or modulate cellular processes to achieve the desired therapeutic effect. This may involve restoring normal cellular function, enhancing immune responses against cancer cells, or targeting specific disease mechanisms.
Gene therapy is arguably the fastest growing therapeutic modality in biopharmaceuticals. On the heels of classic biologics such as monoclonal antibodies (mAb), an increasing number of gene therapies are entering clinical trials and receiving marketing approval. The development of gene therapies has taken longer than that of mAbs for a variety of reasons, including early setbacks and the need for more sophisticated delivery systems.
While biologics such as mAbs can be delivered directly to patients for therapeutic action, gene therapies tend to require delivery systems to safely transport corrective nucleic acids to the required site of action. These delivery systems are most often modified viral vectors. The inclusion of vectors in the final drug product can introduce greater complexity into the processes needed to manufacture gene therapies compared to those typically used for conventional biologics like mAbs.
The biomanufacturing of mAbs and gene therapies requires the use of cellular expression systems, which produce the protein of interest for the final drug product. For mAbs, these systems can come from various species, such as Chinese hamster ovary (CHO) or Escherichia coli (E. coli) cells. For gene therapies, it is much more common to use human cells, such as human embryonic kidney 293 (HEK-293), HeLa or epithelial lung carcinoma A549 (A549) cells.
To derive the final drug product, the product from the cells must be purified, using a series of steps where impurities are detected and monitored until their levels are low enough in the final drug product to meet regulatory requirements. An important class of impurities are host cell proteins (HCPs) (i.e., proteins in cells used to express the protein of interest).
PHCs must be carefully controlled throughout the biomanufacturing process as a critical quality attribute (CQA). Indeed, healthcare professionals can significantly affect the quality, efficacy and safety of the gene therapy product, for example by inducing or enhancing immunogenicity. In addition to process-related impurities such as PHCs, product-related impurities such as those derived from viral vectors must also be carefully controlled.
Historically, the preferred method for analyzing HCPs and other impurities has been ligand binding assays (LBAs), due to their speed, sensitivity, and ease of use. However, although BALs - namely enzyme immunoassays (ELISAs) - are the main regulatory compliant method currently in use, there has been a steady increase in the number of industry regulators requesting that submissions include data detailing the quantification of HCPs obtained with methods orthogonal to ELISA.
The approach that is rapidly emerging as the method of choice for many biopharmaceutical companies is liquid chromatography (LC) coupled with mass spectrometry (MS). In response to this growing need, Alphalyse and SCIEX have developed new LC-MS methods for the HCP characterization of gene therapies and other new complex biologics (1, 2).
These improved methods and workflows address key gaps posed by ELISAs in the analysis of HCPs for gene therapies. Several key issues are repeatedly encountered by industry players with the application of ELISAs for HCP analysis during the development and manufacturing of gene therapies.
The common problem that frequently arises is the specificity of commercially available ELISAs. During cell manufacturing of various types of gene therapies, there are many sources of potential residual contamination. Mammalian expression systems commonly used to generate gene therapy products, such as those involving HEK-293 cells, introduce a multitude of impurities which are secreted along with the product of interest into the cell culture medium. These impurities can include nucleic acids (i.e. DNA, RNA) and lipids, as well as PHCs and other cellular materials. Additionally, the medium may also include other proteins that have been added to enhance and extend its function, such as
